Well, now that we have covered all of the basics of the atom, its structure and, most importantly, the nucleus’ stability, we can look into what happens when an atom is unstable, or how most atoms are radioactive!
PS. Link to part 1 if you need a reminder: PART 1
Radioactive decay and radiation
Back in the 19th century, X-rays, a type of electromagnetic radiation, were discovered. After the monumental discovery, a scientist Henry Becquerel was working on some experiments with a, then unbenknownst to him, a radioactive compound. He conducted the following experiment: he put the compound in a small tray and put a piece of black paper in front, and left it just like that for a while. After some time, he noticed that the compound left marks on the sheet of paper, which were quite visible.
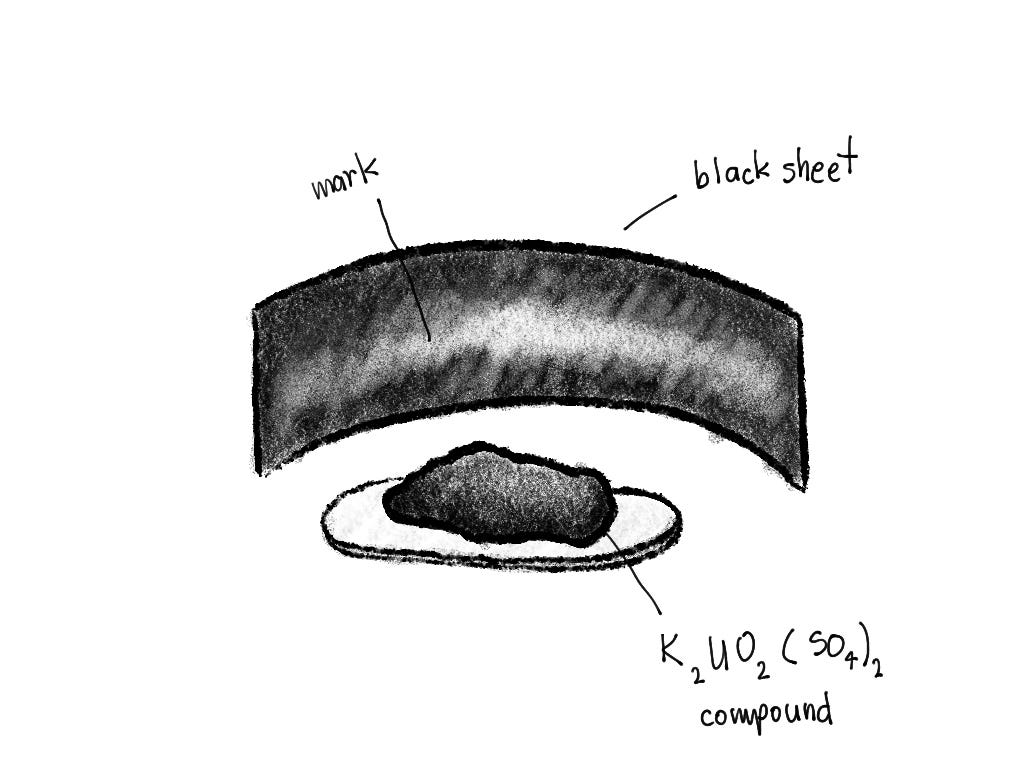
He didn’t know why or how it worked, but we now know this is radioactive decay. The term was coined by Marie Curie, who discovered radium and polonium, two radioactive elements.
A radioactive element is a heavy atom, where the strong nuclear force can’t keep the atom together, or it is excited and contains too much energy. To minimize its energy and regain stability, it releases radiation in the form of 4 types of radioactive decay. In this process, our radioactive atom transforms from one element into another, removing particles that change the chemical makeup of the atom. Radioactivity and decay are the helpers after the war was lost on the stability of the nucleus. When things start falling apart, they pick things up, and save most of the nucleons. By lessening the tension between the nucleons, removing some built-up energy, radioactive decay proclaims peace in the atom.
Disintegration chains
Most commonly, the resulting atom is not perfectly stable during an unstable atom decaying via radioactive decay. So, it keeps on decaying until we reach a stable isotope in a normal disintegration series. However, at specific points, our element can decay by 2 types of radioactive decay, and which branch it will go in a branched disintegration is a probability based on the likelihood of each branch occurring based on experiments!
Isotopes are atoms of the same element with different numbers of neutrons. Neutrons are essential to keeping an atom stable, but they are also a downfall of its stability. An isotope with too many neutrons becomes unstable and can decay. At the same time, an isotope with too few neutrons aggravates protons, rendering the whole atom unstable. However, isotopes are not the only atoms with different radiation or stability levels.
Isomers are atoms of the same element with differing energy levels. As I mentioned earlier in this post, some atoms are radioactive due to high amounts of energy, which makes all the particles jittery. Releasing that energy changes the energy state of the atom into an isomer while still staying the same element. So, how do elements decay exactly?
Types of radioactive decay
Alpha decay
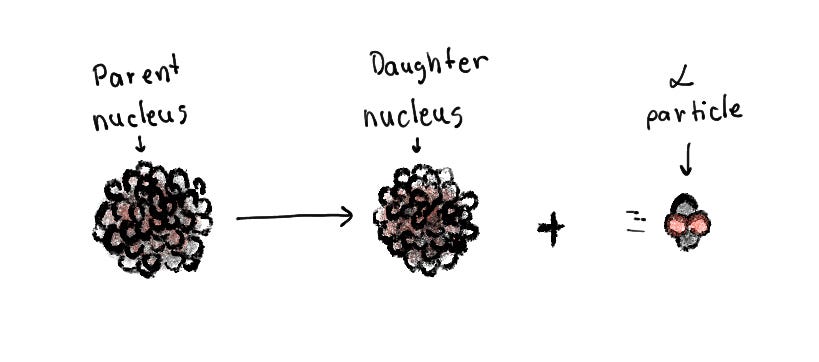
Alpha decay occurs when an unstable nucleus releases 2 protons and 2 neutrons. The parent nucleus decays into a child nucleus and an alpha particle made of 2 protons and 2 neutrons. The alpha particle matches a standard helium nucleus.
How did we discover that? Well, obviously, there was a genius experiment involved. A compound suspected of emitting alpha particles was put inside a tube that had very thin walls, allowing the particles to pass through. That tube was in another tube with thick walls, not allowing the alpha particles to pass through. Between the 2 tubes, electrons were freely floating around. After some time passed, scientists found that the space between the tubes was now filled with helium!
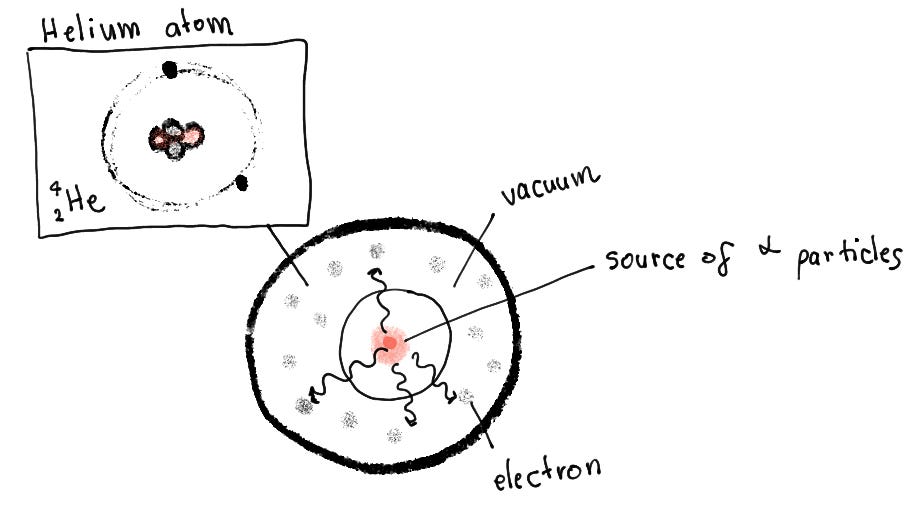
So they knew that the particle contained protons, and subsequently, neutrons were discovered as slightly heavier particles with no charge. During the release of an alpha particle, the element loses 2 protons, becoming an entirely different element in total.
Alpha decay is the dominant way that elements above proton number 83 decay.
Why is this? Because these atoms have too many neutrons and protons to keep stable, hence releasing alpha particles diminishes both neutron and protons numbers, stabilizing the atom.
Beta decay
Beta decay occurs when an unstable neutron converts into a proton and releases an electron and antineutrino, as discussed in the section on neutron instability. This way, the fundamental element also changes, as in alpha decay. This type of beta decay is called beta minus decay since a neutron transforms into a proton. Why does this decay occur? When the number of neutrons against protons is too high, this decay increases the number of protons. It decreases the number of neutrons, making the ratio more manageable for the atom.
However, there is another type of beta decay. It happens when the number of neutrons against protons is too low! So, here, a proton transforms into a neutron, similar to the previous type. One of the up quarks inside a proton turns into a down quark, mediated by our boson particle. It quickly decays into a positron and a neutrino. A positron is the arch-version of an electron. It has the same mass and spin but an opposite charge. When a positron and an electron come into close contact, they annihilate each other! We usually dub the positron as antimatter (more about matter and antimatter in another post).
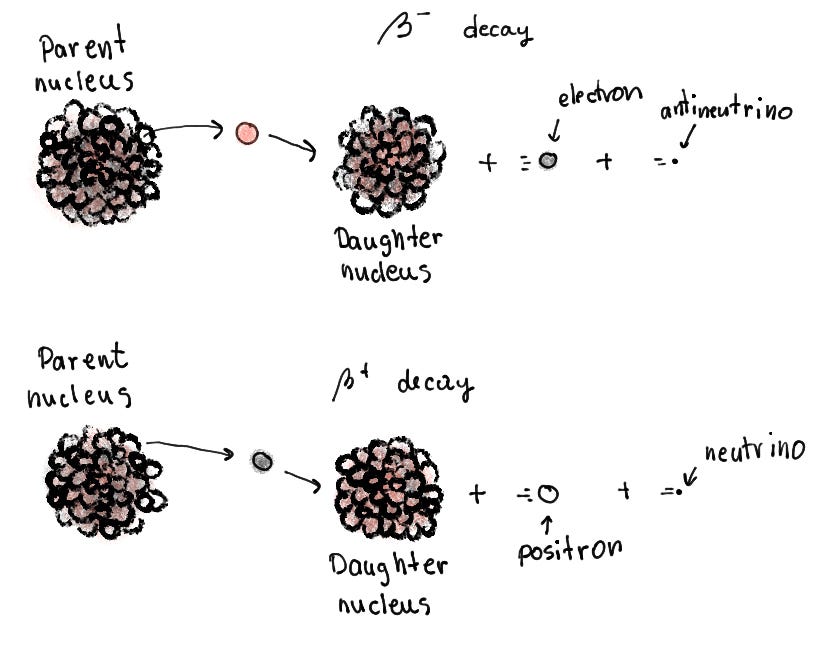
Wait, I think I forgot to explain a particle… Ah, it was the neutrino! It is a crucial particle to beta decay, and neutrinos are actually found everywhere in the universe!
The elusive neutrinos
Neutrinos are particles with almost no mass (extremely close to 0) and almost no charge. They are usually produced in very violent astronomical situations like in the cores of stars, in supernova explosions, orbiting black holes, etc. Neutrinos are specific in their nature because they interact with matter extremely rarely, making them the most abundant particle in the universe! However, this also makes their detection extremely difficult.
These elusive particles were first theoretically postulated by physicist Wolfgang Pauli in the 1930s. Energy and angular momentum weren’t conserved in the beta decay reactions, and both need to be conserved if the universe would exist! So, Pauli proposed that there must exist a particle which doesn’t interact with other particles and is neutral in terms of charge, everything would be conserved! And so, in 1955, the first antineutrinos (remember, the antimatter version of the neutrino) were detected in a nuclear reactor.
Detection of neutrinos
How were they detected? As we know, in beta decay, an antineutrino is released. Those antineutrinos passed through a liquid in the detector where if a antineutrino interacted with a proton, it would produce a positron (antimatter version of the electron) and a neutron. The positron quickly connects with the electron, and sine one is antimatter and the other matter, they are quickly annihilated, but in the process they release gamma waves, and that is what scientists detected!

Figure 5: detection of antineutrinos
However, the first natural neutrino was detected in 1965. Deep in a mine, scientists put a large tank filled with perchloroethylene, a chemical compound that contains Chlorine 37. When neutrinos travel from the sun all the way to Earth and penetrate into the tank, some interact with the chlorine to produce Argon 37. And, the researchers detected Argon 37! But, there was a problem.
The solar neutrino problem
The researchers measured only a third of the neutrinos which would theoretically come from the sun. Scientists knew that there are 3 types of netrinos: electron neutrinos, muon neutrinos and tau neutrinos. Like quarks, each has its specific properties. This is known as the solar neutrino problem. However, scientists think they have solved the problem. They discovered that neutrinos oscillate a lot between types, so we only receive a third of the neutrinos from the sun, because other neutrinos convert to other types which don’t reach the detectors. These discoveries infer that neutrinos have slight mass, even though thus far it was thought they were massless.
Scientists actually think that there might even be a fourth type of neutrino which could explain dark matter! But, that is for a future post when we discover something, since up until now everything has just been theoretical 😉
Gamma decay
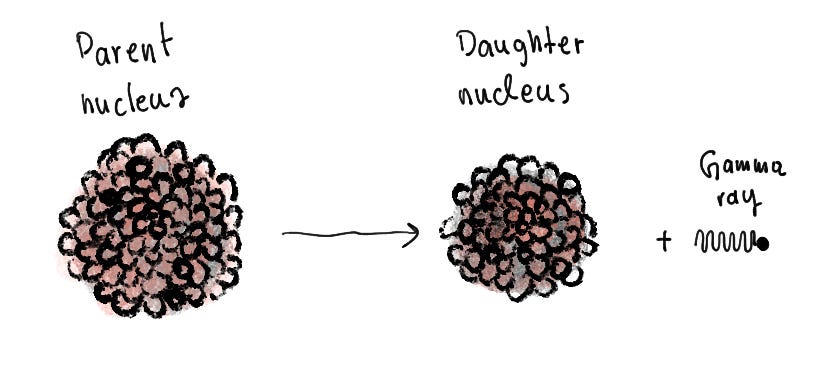
Gamma decay is a type of radioactive decay where no particle is emitted and the parent radioactive atom stays the same element. The parent atom emits a gamma ray, or a super high energy photon. This lowers the energy levels of the parent atom, rendering it more stable.
Electron capture is a type of decay where a proton inside the nucleus “captures” an electron which is closest to the nucleus, transforming into a neutron and releasing a neutrino. Since there is now an empty spot for an electron in the innermost orbital, an electron from a higher energy level comes in, during the process releasing an X-ray.
As we can notice from the 4 types of decay, the first two (alpha and beta) correspond to isotopes, and how different elements decay into different isotopes of different elements. Meanwhile, the third type (gamma) corresponds to isomers, transforming into the same element of a different energy level.
The life of a radioactive atom
Nothing lasts forever. The principle applies especially to radioactive elements, which decay after a while to preserve as much as possible, peace between nucleons. But, there is always a defined time until a peace treaty is resolved. This time is called a half-life of a radioactive atom. It is the amount of time that it takes half of all the radioactive samples in the population to decay into more stable variants. The following graph depicts the half-life curve of a radioactive atom.
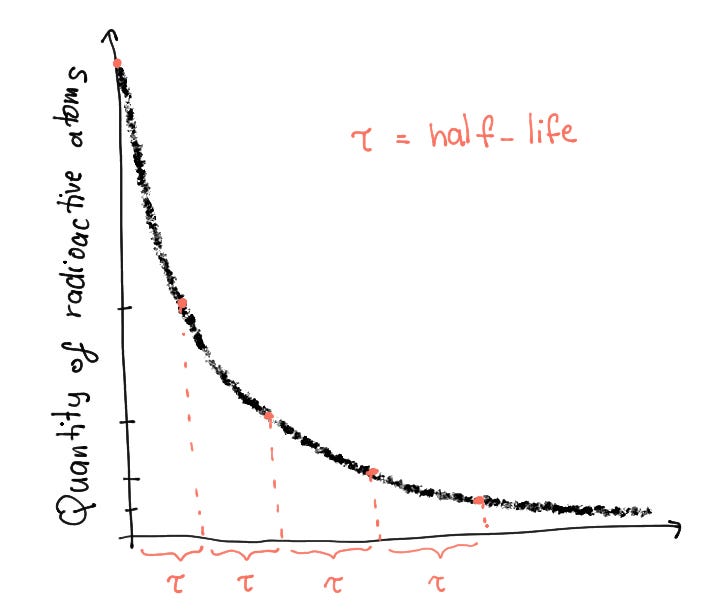
As you can probably infer, the half-life function is an exponential function, meaning that as we move by one unit (a half-life), half of every consecutive part of the original population of the radioactive element decays. The shorter the half-life, the more radioactive the element is!
Nuclear reactions
After discussing so many properties of nuclei, let’s put it all together, and analyze 2 nuclear reactions where they bring life and opportunities, but also death and destruction.
Nuclear fission
So, as you can imagine, during radioactive decay, energy is released. As a atom decays into a parent and daughter element, particles or energy is released, and part of the mass released during the decay turns into energy. As uranium decays, it can release a very large quantity of energy if enough grams of uranium decays naturally. However. There is another way.
In an experiment in 1938. scientist Lise Meitner fired barium nuclei at uranium nuclei, and he couldn’t separate the barium from the uranium anymore. No flood of radioactive particles. It appered that the uranium nucleus was split in half. This is the first evidence of nuclear fission! This process released much, much more energy than natural radioactive decay, so humans used nuclear fission in nuclear reactors and weapons like the nuclear bomb.
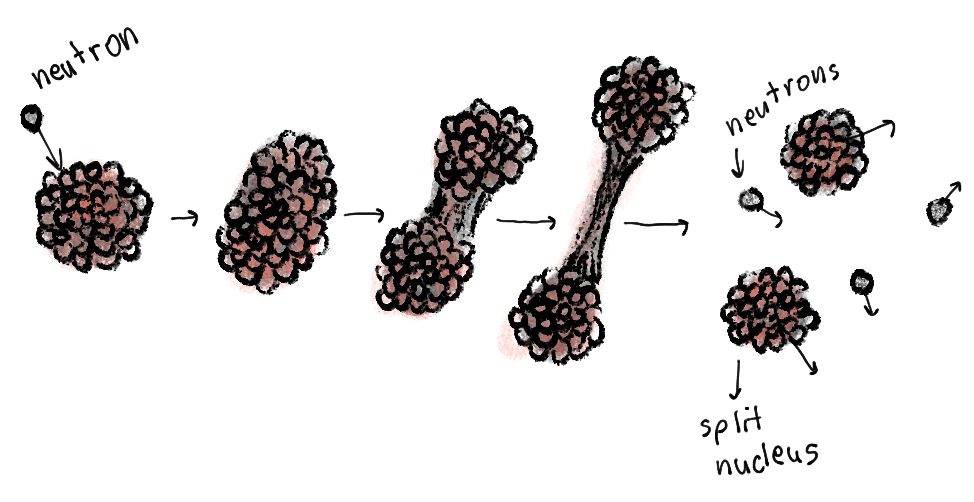
Figure 8: the process of nuclear fission
How does one aquire enough energy from these processes to power a nuclear reactor or a bomb? Well, once one neutron bombards one uranium atom, it splits and ejects “lost” neutrons which hit other uranium atoms and in this fashion a chain reaction is induced, either in a controlled (nuclear reactors) or explosive (bomb) manner.
But, why doesn’t nuclear fission just occur naturally (in most cases)? Well, you can imagine every radioactive decay as an “energy path” which can contain bumps and hills. When radioactive decay occurs, the bump is so small that very little energy is required to begin the reaction. However, the bump for fission is much, much higher, so particles literally need to smash into one another so enough kinetic energy is converted so nuclear fission can occur.
Nuclear fusion
The second type of nuclear reaction is nuclear fusion! This type of reaction is the very reason why any of us are alive on Earth today and why the whole universe exists in such wonder as today.
Nuclear fusion is a nuclear reaction where particles fuse together to form more complex elements. So, it is the opposite of nuclear fission. As of now, fusion is not yet possible on Earth on a scale which would produce energy, but scientists are working hard on it!
The energy of the stars
The combining of smaller particles to form different elements releases less energy that nuclear fission, however on very large scales, meaning there are massive amounts of particles, the energy increases drastically! The Sun is around 1.99 * 10³⁰ kg. Each gram contains 4.5 * 10²³ protons! Now, not every bit of the Sun can be used for fusion, only the very core. So, our Sun has around 10 * 10⁹ years to live on its supply of protons. The energy of the Sun produced by fusion is so great, one hour of fusion can supply humanity for around 2.3 billion years! That is insane. Due to our current technological standing, we aren’t able to reproduce fusion on Earth or even capture a significant fraction of that energy, but someday we just might.
Stars live by fusing elements together. We start off with hydrogen, then helium, and we progress on to heavier elements all the way to iron. Lower elements (like hydrogen, helium,…) when fused together produce more energy than higher elements (carbon, oxygen,…), and iron is the final element in the fusion cycle of stars. Why? Because Iron is too heavy to produce more energy, rather it consumes energy and it can’t be fused further in the cores of even the most massive stars.
The fundamental fusion process is called the proton-proton chain. Let’s explore it.
Proton-proton chain
So, in a star’s core, temperatures are extremely high (in the Sun its around 15 million degrees Celsius), and atoms aren’t kept together. The individual particles and nuclei of atoms are floating around in an ionized state called plasma. Plasma can be thought of as another state of matter with its own unique properties.
So, 2 lonely protons collide together to form a deuteron, which is a heavier version of hydrogen, made of a proton and neutron. In the process, a positron and electron neutrino are released (beta plus decay, the proton turned into a neutron). This is where we find our electron neutrinos from the Solar neutrino problem! Now, the deuteron fuses together with another straggling proton. Now they form a Helium 3 nucleus, containing 2 protons and a neutron, releasing a gamma ray in the process. Finally, another Helium 3 nucleus approaches (formed by the same process described so far), and together they form a proper Helium 4 nucleus, containing 2 protons and 2 neutrons. In the process 2 protons are released, ready for a new proton-proton chain.

Figure 9: proton-proton chain
And with this process, every object and structure you see in the universe was created.
And, we end at the beginning. With quarks coming together in the early universe, forming protons and neutrons, the universe cooling down enough to form atomic nuclei, the battle between the nucleons fought every single moment, when electrons were finally able to join the nuclei and form atoms, which in turn created every beautiful thing around us.
I hope you learned something new about the wonderful universe around us, and to never underestimate the complexity and power of something so small as the nucleus.
REFRENCES
- Chemistry The Central Science by Theodore L. Brown H. Eugene LeMay Jr Bruce E. Bursten Catherine J. Murphy Patrick Woodward Matthew E. Stoltzfus, Pearson, 14th edition, chapter 21 on Nuclear Chemistry
- Understanding physics Volume 3 by Isaac Asimov, Barnes&Noble books, 1996, chapters 7, 8, 9, 11, 12, 14
- University Physics with Modern Physics by Hugh D. Young, Roger A. Freedman, A. Lewis Ford, Pearson, 15th edition, chapter 43
- https://www.scientificamerican.com/article/what-is-a-neutrino/
- https://www.space.com/what-are-neutrinos
- https://neutrinos.fnal.gov/whats-a-neutrino/
- https://www.youtube.com/watch?v=lUhJL7o6_cA